It is still not fully understood how the enzyme beta-lactamase in bacteria carries out this process. Some important details of the reaction mechanism are still controversial. However, the understanding of this mechanism could help develop new antibiotics. Leighton Coates and co-workers from the Oak Ridge National Laboratory, USA, have now studied in detail the mechanism of a β-lactamase enzyme using neutrons at the Forschungs-Neutronenquelle Heinz Maier-Leibnitz (FRM II).
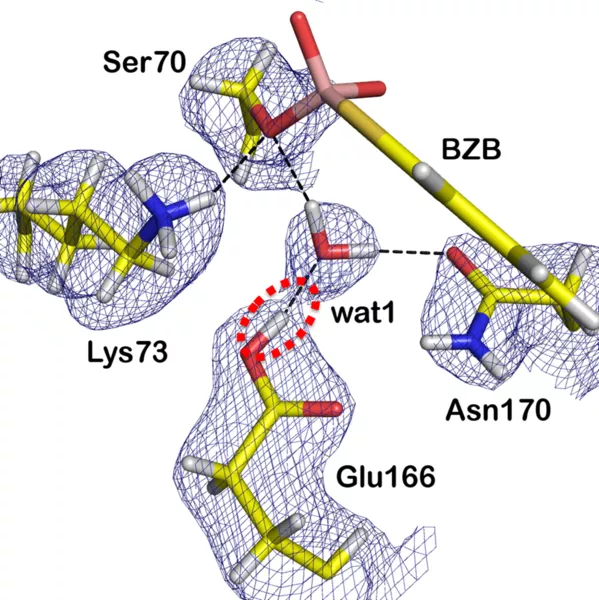
They used the diffractometer BioDiff, which is operated jointly by Andreas Ostermann (FRM II, Technische Universität München) and Tobias Schrader (Forschungszentrum Jülich). In order to locate the hydrogen atoms in the enzyme using neutron diffraction, the researchers replaced all hydrogen atoms in the enzyme by its heavier isotope, the deuterium atom. Thus, the positions of the deuterium atoms in the active site of the enzyme could be determined, while the enzyme is bound to an antibiotics’ analogue.
During the reaction in the active site, a proton acceptor temporarily takes a proton. So far there have been two conflicting hypotheses, which residue acts as the general base in part of the mechanism. Numerous studies have suggested it is the Lys-73 residue, while other studies suggested the Glu-166 residue. The neutron scattering experiments at BioDiff, complemented by high-resolution X-ray analysis, however, clearly showed that the Glu-166 acts as a proton acceptor.
Original Publication:
Stephen J. Tomanicek, Robert F. Standaert, Kevin L. Weiss, Andreas Ostermann, Tobias E. Schrader, Joseph D. Ng und Leighton Coates
Neutron and X-ray Crystal Structures of a Perdeuterated Enzyme Inhibitor Complex Reveal the Catalytic Proton Network of the Toho-1 β-Lactamase for the Acylation Reaction
Journal of Biological Chemistry, 288(7):4715-4722, 2013